
5.85g of NaCl is dissolved in 1L of pure water. The number of ions
5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is

PPT - Solutions PowerPoint Presentation, free download - ID:528392

WO2020102346A1 - Methods of treatment for cystic fibrosis - Google Patents

Malayalam] 5.85g NaCl is dissolved in 1L water. The number of ions of
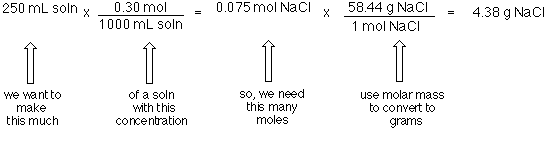
Solutions
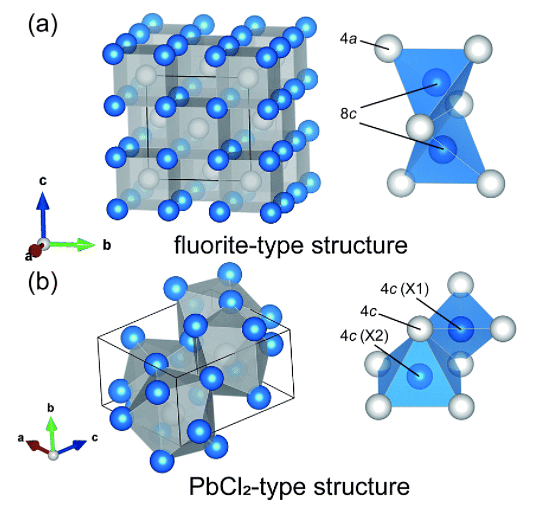
Lead II Chloride Formula: Properties, Structure, Examples
What is the molarity of sodium chloride in a solution that is 13.0% by mass sodium chloride and that has a density of 1.10g/ml? - Quora
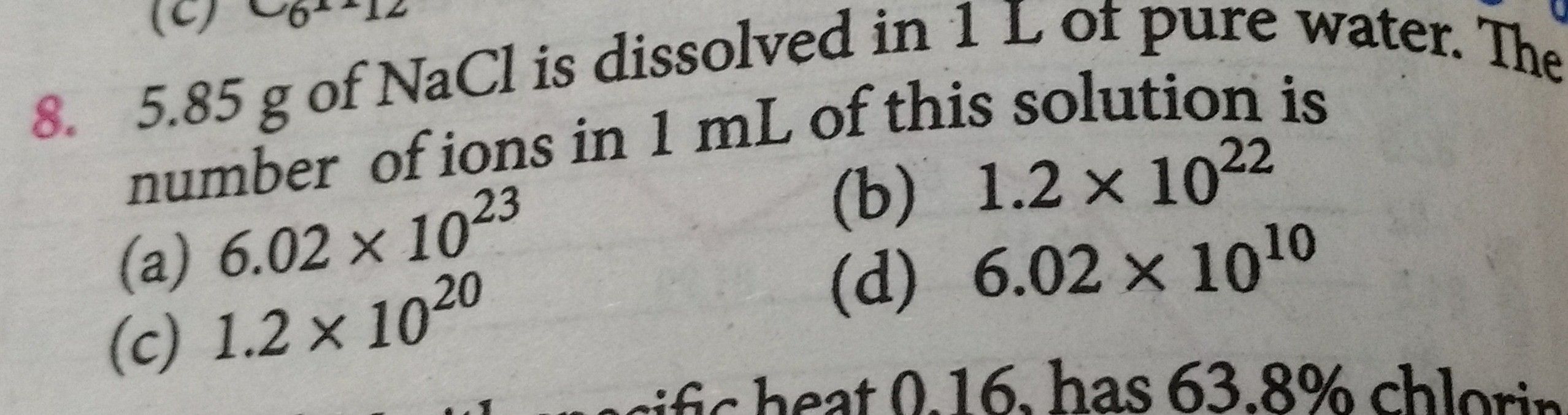
Questions and Answers of Some Basic Concepts Of Chemistry Mole Concept of CBSE Science Chemistry - TopperLearning

INORGANIC SYNTHESES Volume XX

1.3 Concentration and Titrations calculations..docx - Concentration and Titrations: 1. Concentration: In the laboratory the majority of reactions







)

