
Understanding The FDA's Current Focus On Risk Evaluation And
lt;p>The FDA recently asked for comments about how the government handles vendor change requests from drug sponsors with risk evaluation and mitigation strategies. So, we asked a REMS expert to help us understand why the agency is focusing on the broad-reaching program and what it could mean for drug manufacturers with REMS products in their portfolios.</p>

US FDA approvals bounce back in 2023, sparking hopes of a biotech
FDA issues final guidance on developing drugs for COVID-19
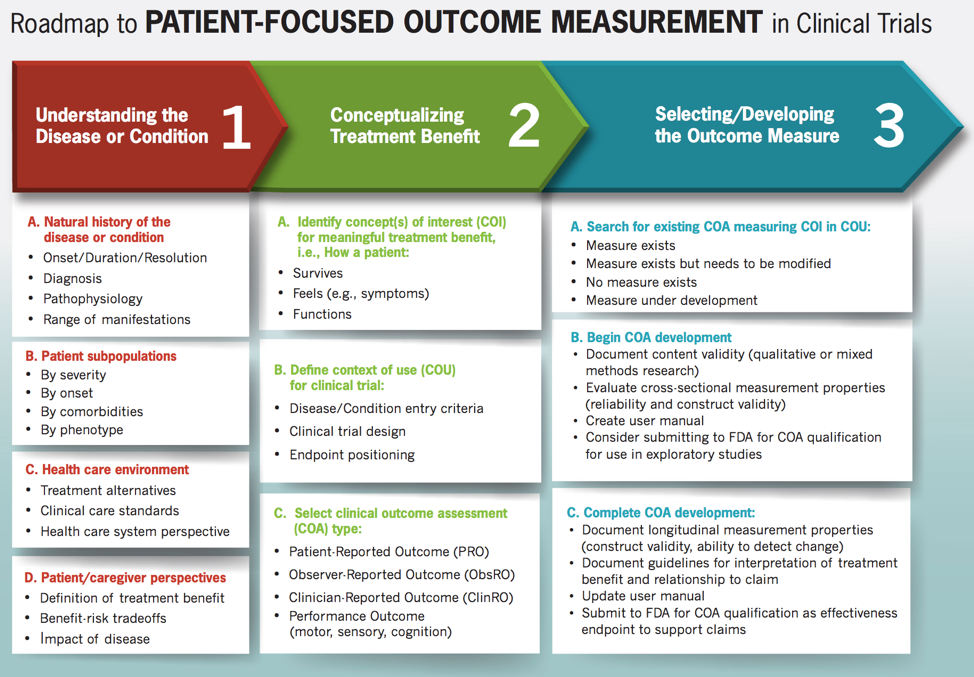
FDA's Roadmap to Patient-Focused Outcome Measurement in Clinical

2021 Dietary Guidance to Improve Cardiovascular Health: A

FDA's Recent Benefit Risk Assessment Guidance Explained

HIGH-RISK SERIES Efforts Made to Achieve Progress Need to Be

Understanding The FDA's Current Focus On Risk Evaluation And
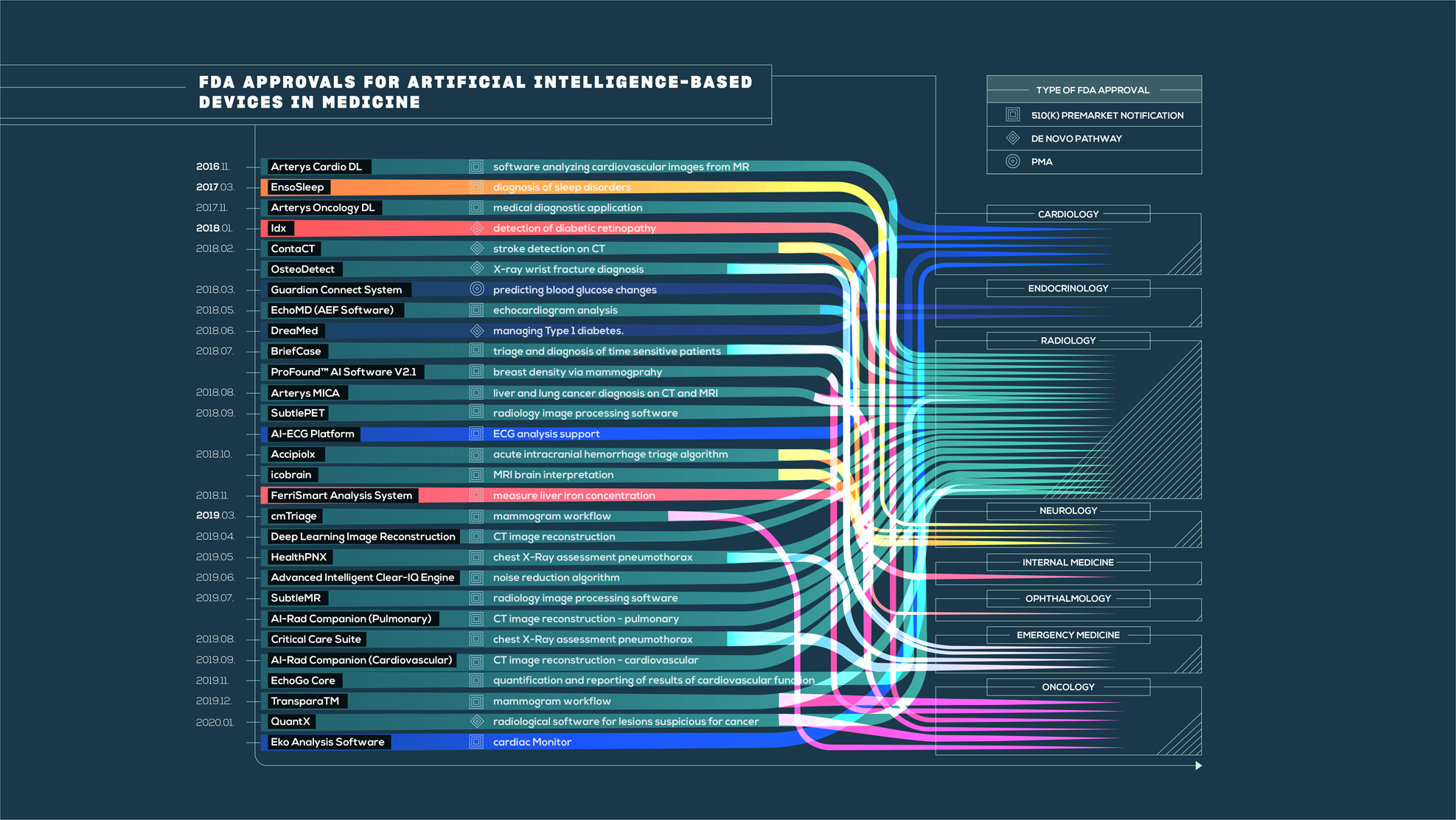
The state of artificial intelligence-based FDA-approved medical

U.S. FDA allows abortion pills to be sold at retail pharmacies

Standardized Metrics for Better Risk Management: The Right Data at

FDA Proposes Risk-Based and Remote Inspection Strategies in New

FDA curbs unfounded memory supplement claims - Harvard Health

FDA: How Pharma Companies Are Unknowingly Introducing Risk Into









