A 1.50 mL sample of a sulphuric acid solution from an automobile storage battery is titrated with - YouTube
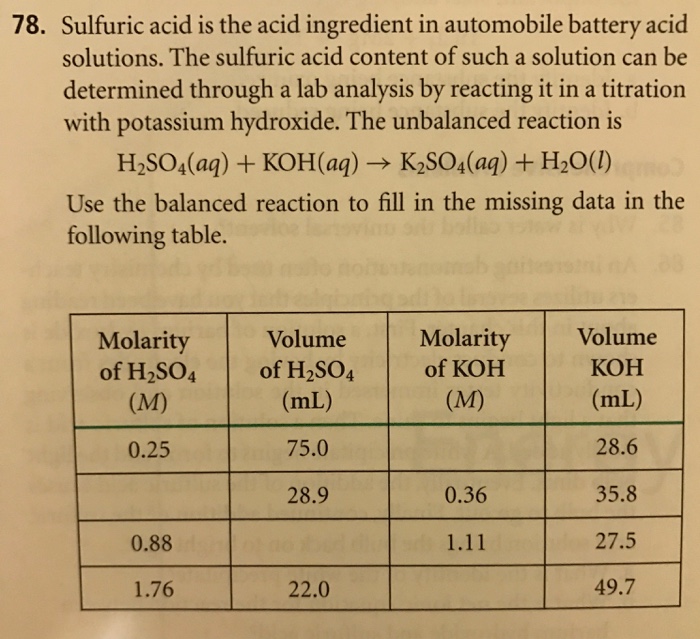
SOLVED: A car battery acid was made by dissolving 573.6 g of H2SO4 in 1.2 Kg of water. The resulting solution has a density of 1.329 g/cm3. Calculate the molality of the

Battery acid is 4.22 M aqueous H, SO4 solution, and has density of 1.21 g cm-3. What is the molality of H,SO,? H = 1, S = 32, 0 = 10 (m = 5.298 mol kg-1)

Battery acid is 4.27 molar h2 s o4 and has a density of 1.25 gram per ml what is the molality of h2s o4 in - Brainly.in

11. Battery acid is 4.27M H250, and has density of 1.25g/ml. What is the molality of HySO. In the solution?

10) Battery acid is 4.22 M aqueous H2SO4 solution, and has density of 1.21 g cm-?. What is the molality of H2SO.? H = 1, S = 32, 0 = 16 (m = 5.298 mol kg )

and 90 g of water have been mixed. A battery acid contains 24.5% by mass of H2SO4. What is the molality of the solution? A sample of sodium hydroxide weighing 0.48 g

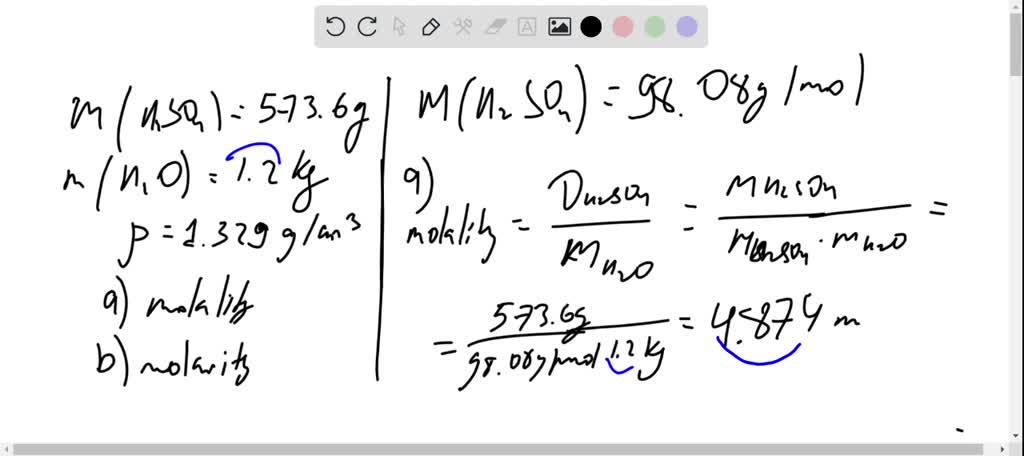
The molarity of sulfuric acid in a fully charged car battery is 5.2 m. when fully discharged the molarity - brainly.com

11. Battery acid is 4.27M H250, and has density of 1.25g/ml. What is the molality of HySO. In the solution?








:max_bytes(150000):strip_icc()/car-battery-recycling-container-with-warning-notices-battery-acid-flusco-household-waste-recycling-centre-cumbria-uk-121814398-57a4e5055f9b58974a7355d8.jpg)