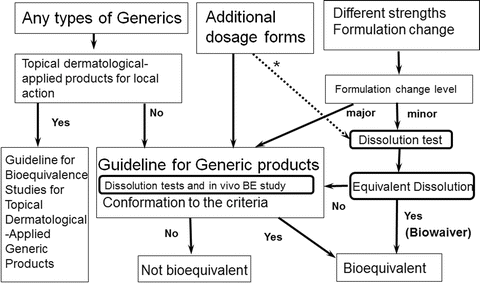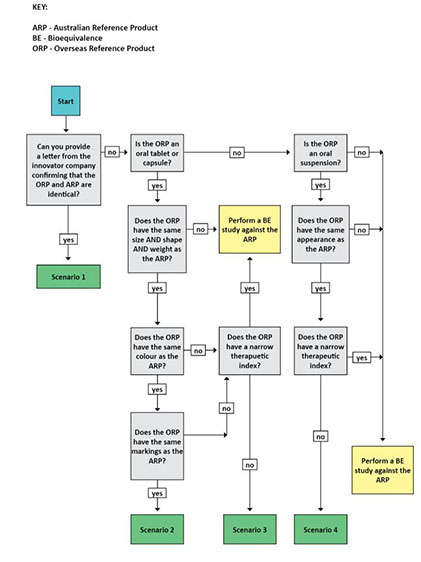
15.6 Choice of the reference product for bioequivalence of generic medicines | Therapeutic Goods Administration (TGA)

Current regulatory scenario and alternative surrogate methods to establish bioequivalence of topical generic products - ScienceDirect

GENERIC DRUGS: Guidelines for bioequivalence studies: Vishwakarma, Pushpendra Kumar: 9783639343779: Amazon.com: Books
Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countrie

PDF) ASEAN GUIDELINES FOR THE CONDUCT OF BIOAVAILABILITY AND BIOEQUIVALENCE STUDIES | jessie wu - Academia.edu

PDF) Implementation of Bioequivalence Studies for Approval of Generic Drug Products in Sudan: Current Status | abubakr Nur - Academia.edu

PDF) International Guidelines for Bioequivalence of Systemically Available Orally Administered Generic Drug Products: A Survey of Similarities and Differences