
Does The Difference In Structure Make Graphite Soft But Diamond Hard?
Diamond Graphite

Why graphite is a conductor but not a diamond?
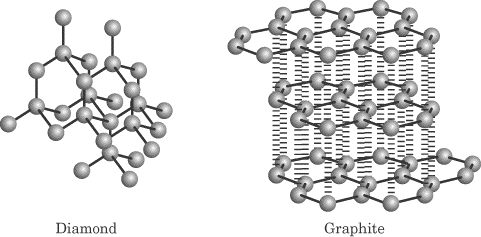
Properties of solids

Searching for the Origin of the Battery - Graphite Battery LAB

Look the diagram and answer the following questions:i) What of structure do diamond and graphite have?ii) Why are diamonds used in cutting tools?iii) Why is graphite used in electrical circuits?iv) Name the
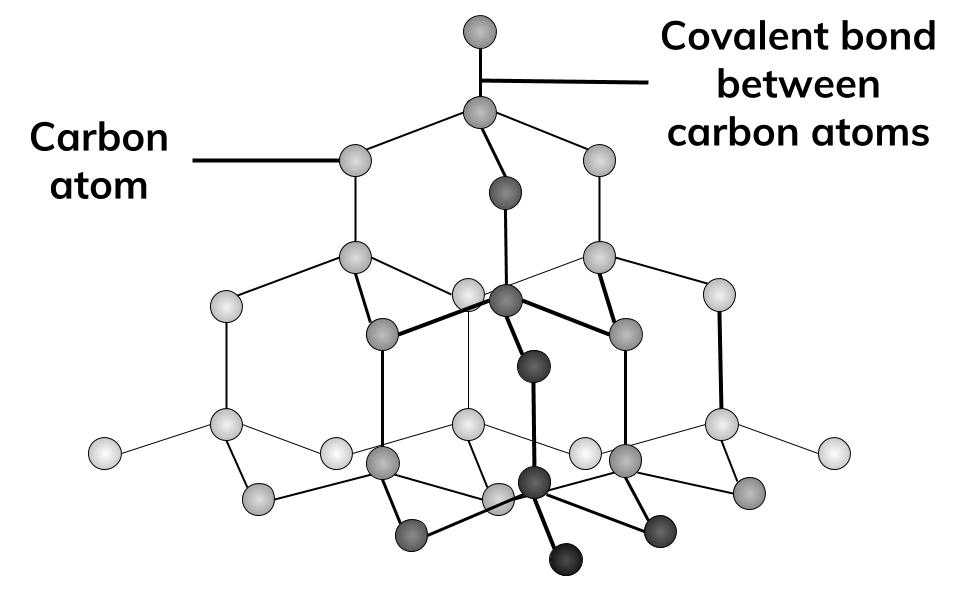
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

Why is diamond hard while graphite is soft?
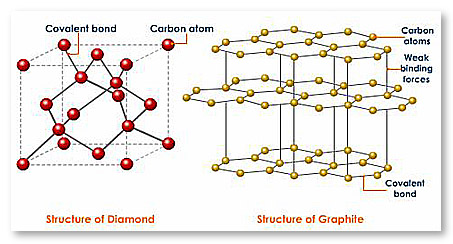
Hardness of Diamond - It's Hardest and NOT Toughest substance
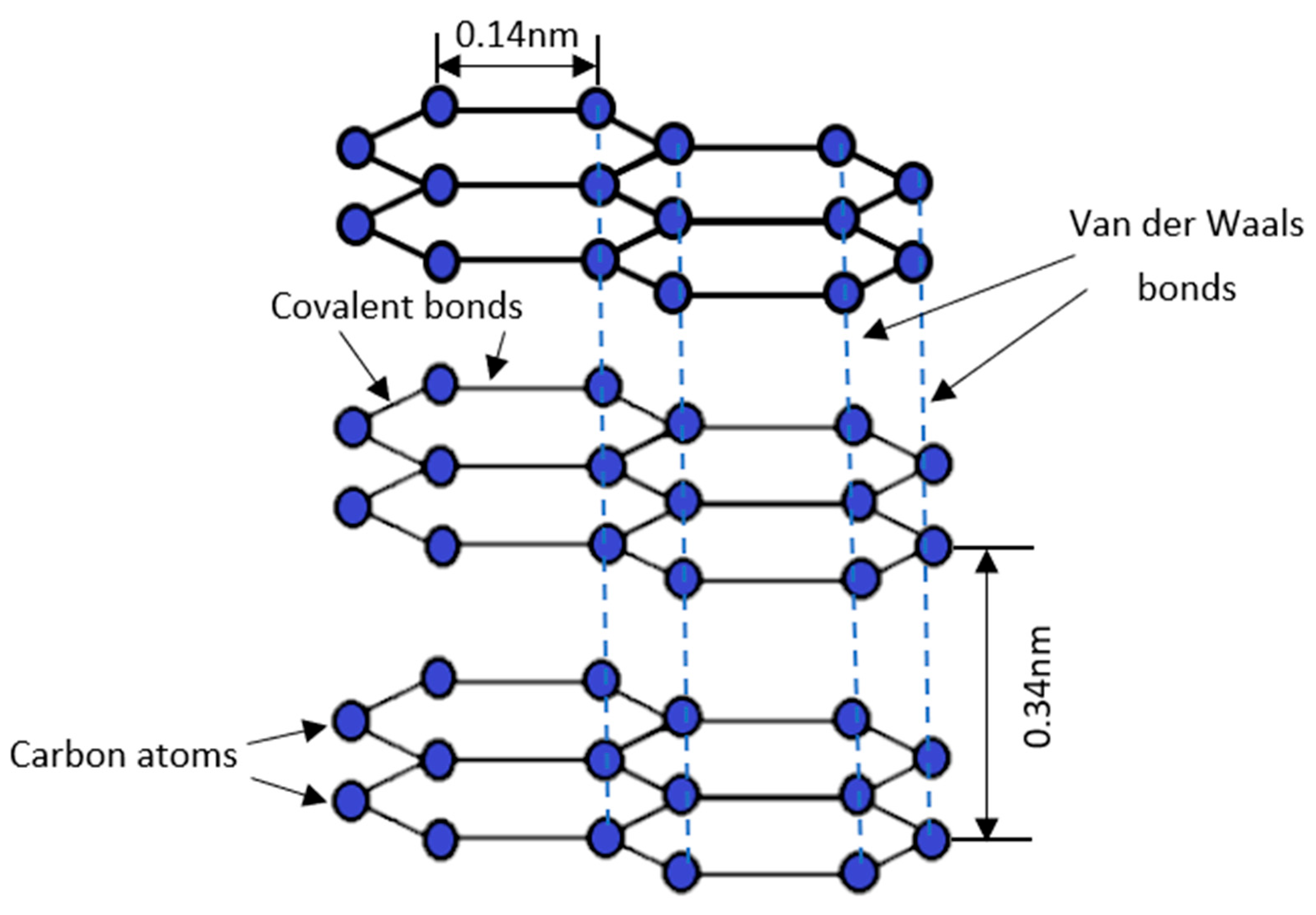
Materials, Free Full-Text
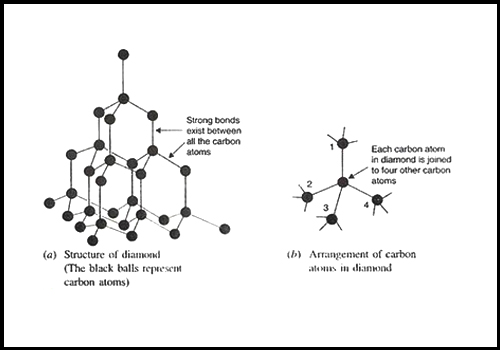
Describe why diamond is hard and graphite is soft?

Extreme pressure? Diamonds can take it

Crystal structures of diamond and graphite.

Does The Difference In Structure Make Graphite Soft But Diamond Hard?

Giant Covalent Structures, SL IB Chemistry Revision Notes 2025








:format(webp):quality(70)/https%3A%2F%2Fmedia.topito.com%2Fwp-content%2Fuploads%2F2019%2F02%2Fsticker-shrek-voiture.jpg)
