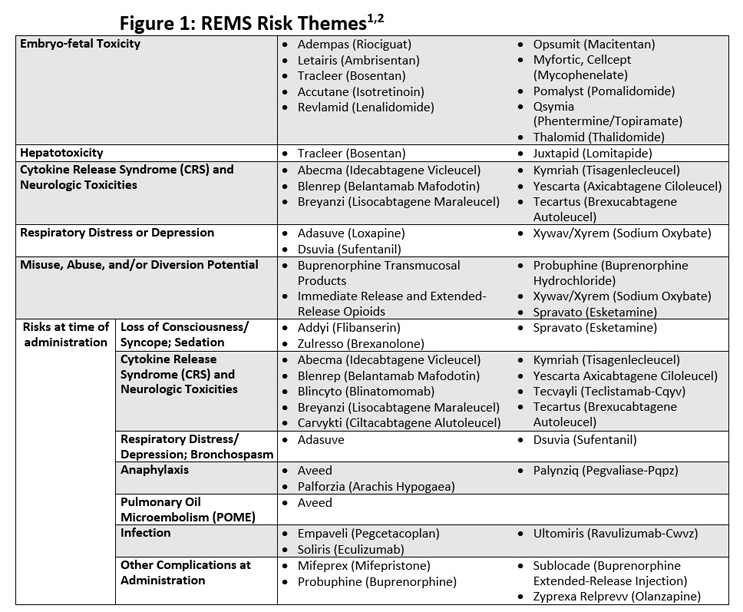
Avoid Launch Delays By Planning For An FDA-Required REMS Risk
lt;p>Picture this: The FDA accepts a manufacturer's NDA, and the manufacturer plans for its impending launch. But shortly before the anticipated approval, the FDA notifies the manufacturer that a Risk Evaluation and Mitigation Strategy (REMS) program is required to market the product. Now what?</p>

Have REMS Programs Reached Stable State 7 Years Post-Approval?

FDA Regulation of Prescription Drugs

White Paper, Missed Opportunities When Developing a REMS Program

Improving Risk Evaluation and Mitigation Strategy

REMS Consulting
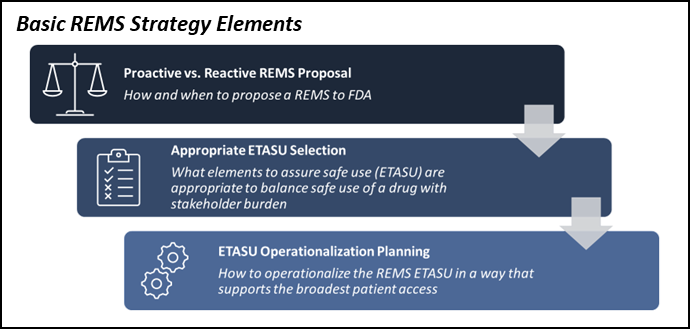
Avoid Launch Delays By Planning For An FDA-Required REMS Risk Evaluation and Mitigation Strategy

REMS Modernization Can't Wait A Call to Action

White Paper, Missed Opportunities When Developing a REMS Program
From Our Perspective, A Two-Part Series: Risk Evaluation and Mitigation Strategies (REMS) Program
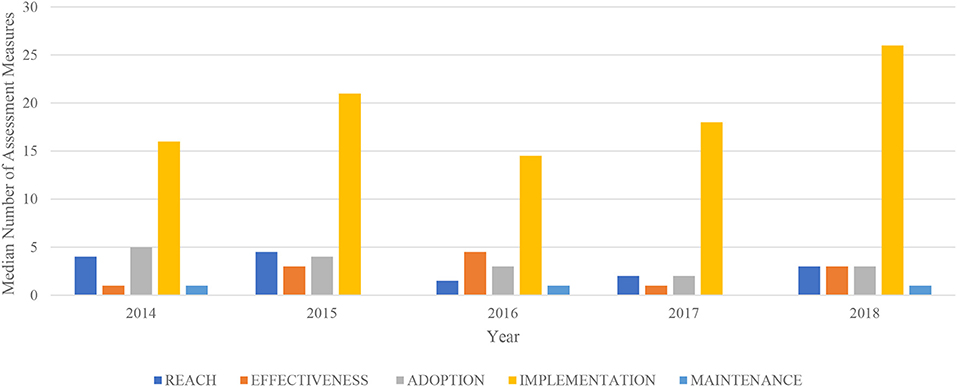
Frontiers Adaptation for Regulatory Application: A Content Analysis of FDA Risk Evaluation and Mitigation Strategies Assessment Plans (2014–2018) Using RE-AIM

Responding to the opioid crisis in North America and beyond: recommendations of the Stanford–Lancet Commission - The Lancet
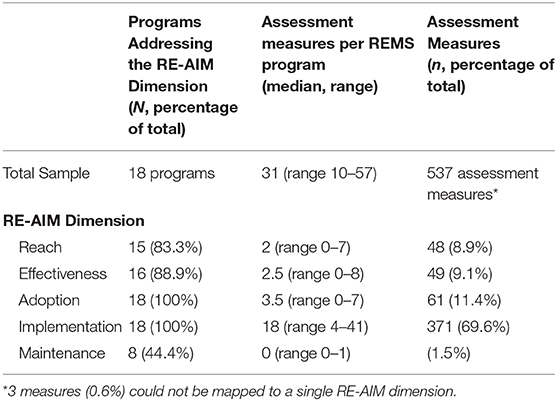
Frontiers Adaptation for Regulatory Application: A Content Analysis of FDA Risk Evaluation and Mitigation Strategies Assessment Plans (2014–2018) Using RE-AIM