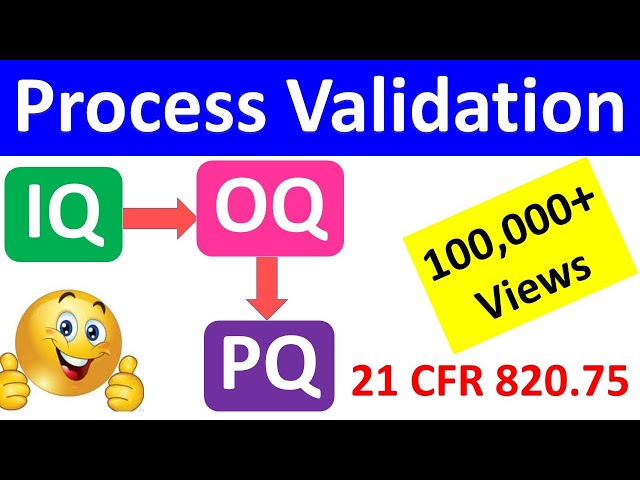
IQ OQ PQ, Process Validation, Equipment Validation
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.

Validation Qualifications IQ OQ PQ : PresentationEZE

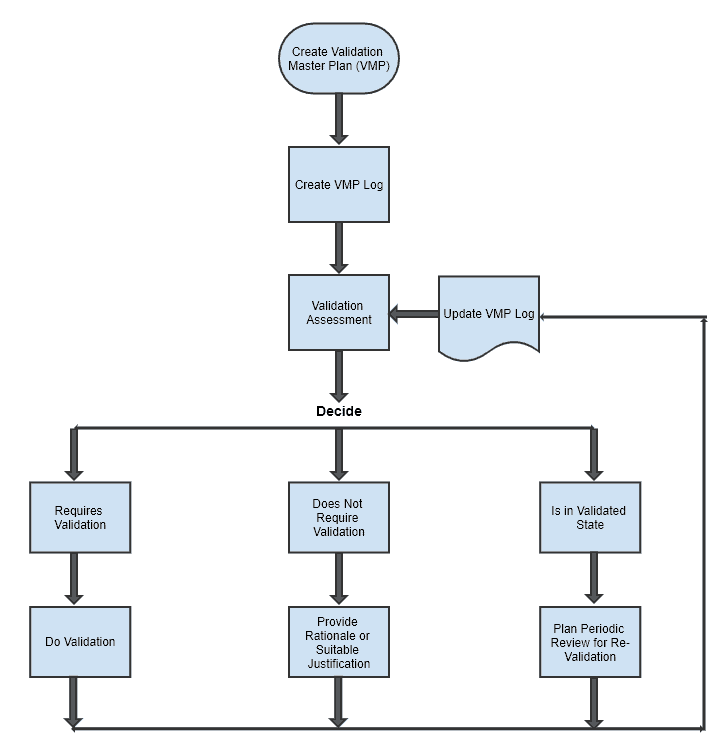
What is a master validation plan
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP
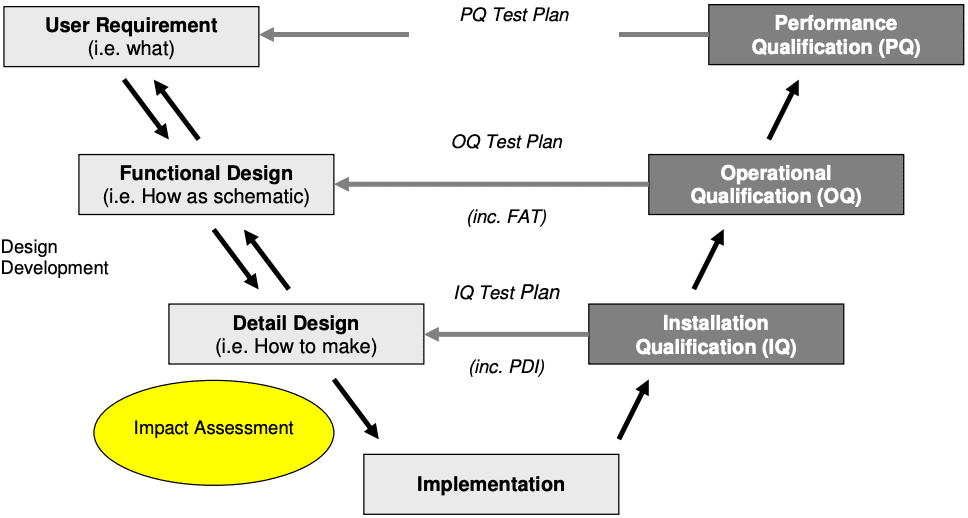
What are IQ OQ PQ? Why are they critical to the Pharma Industry?
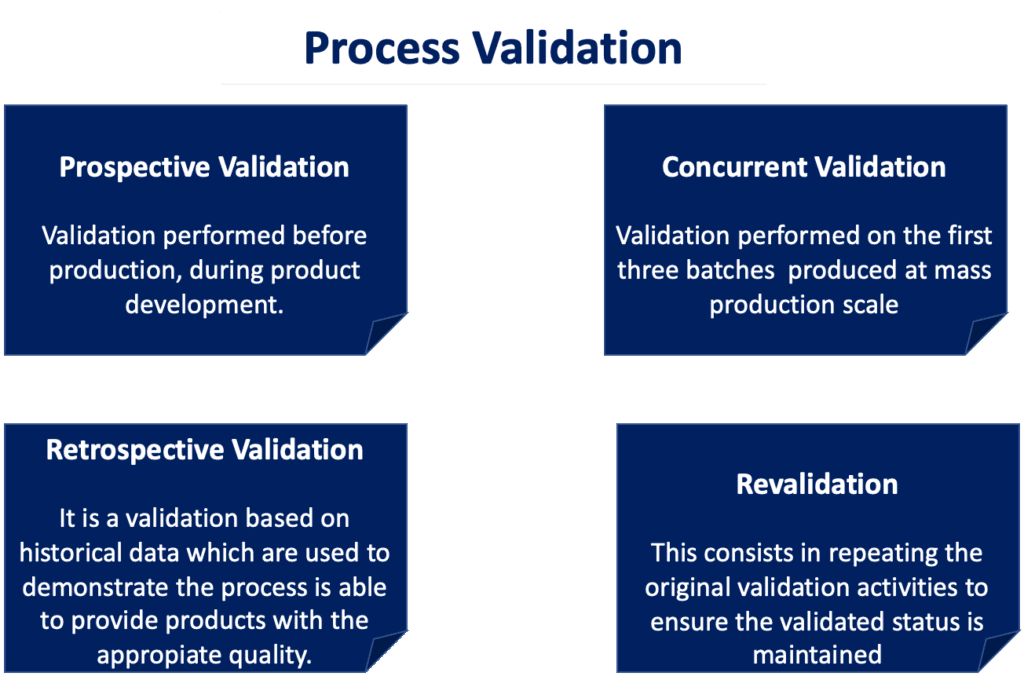
Process Validation for Medical Devices: Overview of FDA Requirements

IQ, OQ, PQ — A Guide to Process Validation, by IZiel Healthcare
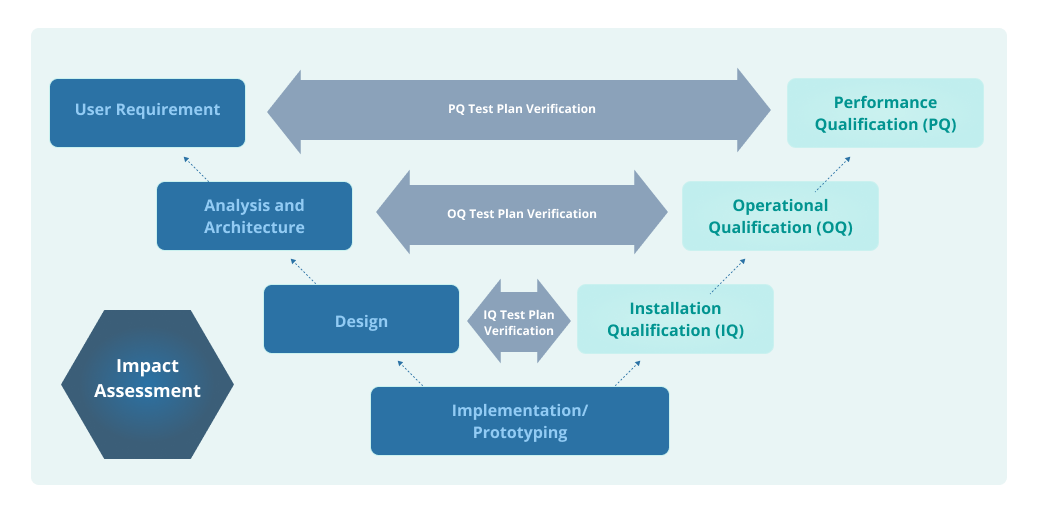
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries

IQ-OQ-PQ-Combined - Just follow the integral SOP to customize.
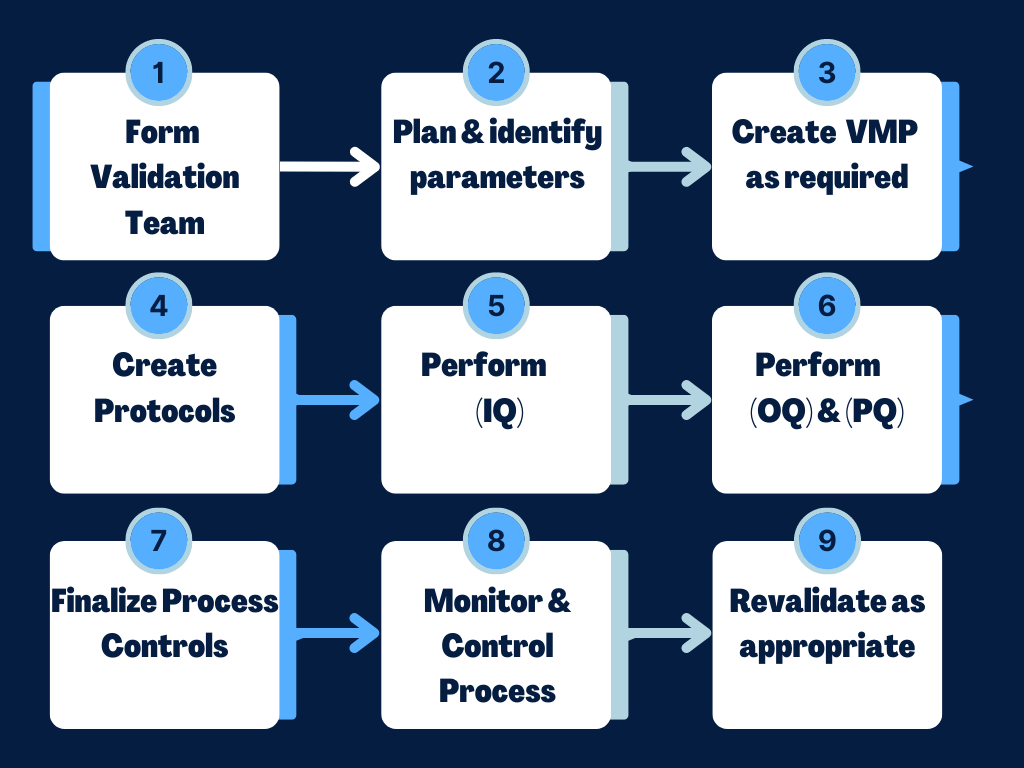
Fast Track ISO 13485 Process Validation Explained for your Medical Device

What Are IQ OQ PQ, The 3 Q's Of Software Validation Process