
Explain the structure of Graphite.
Click here:point_up_2:to get an answer to your question :writing_hand:explain the structure of graphite
Click here👆to get an answer to your question ✍️ Explain the structure of Graphite-
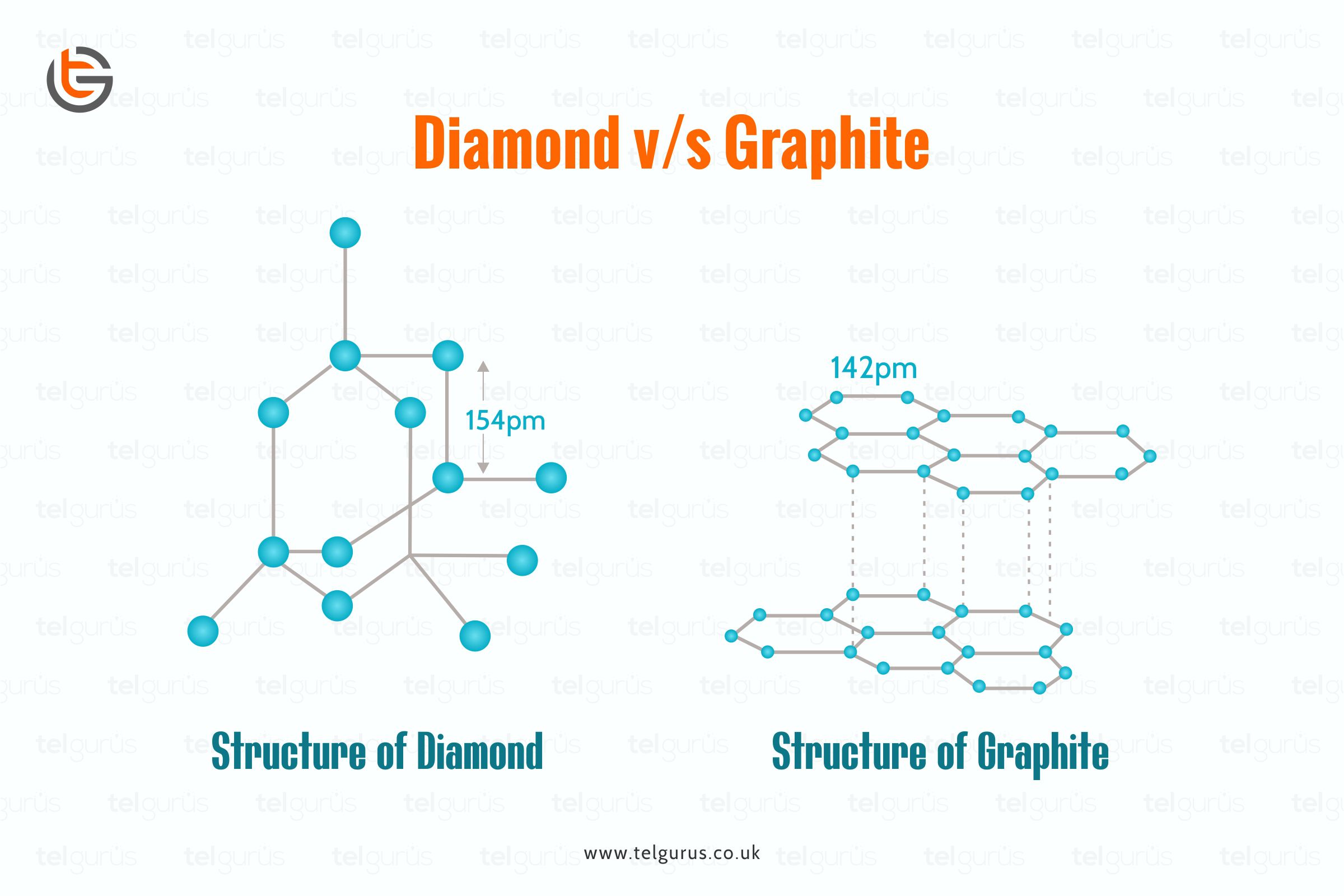
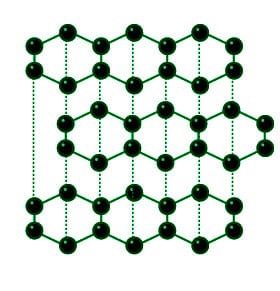
In Graphite each carbon atoms is united with three surrounding carbon atom through covalent bond and forms a sheet-like structure- These sheets or layers are stacked one above the other to form three dimensional structure-Each layer is made up of hexagons there is no covalent bonding between the layers- These layers are held together by weak Vander Wall-s physical forces- Hence these layers can slide over one another

Crystal structure of graphite. The unit cell is shaded in green. ( A )

Graphite - Definition, Structures, Applications, Properties, Use with Videos and FAQs of Graphite
Describe why diamond is hard and graphite is soft?
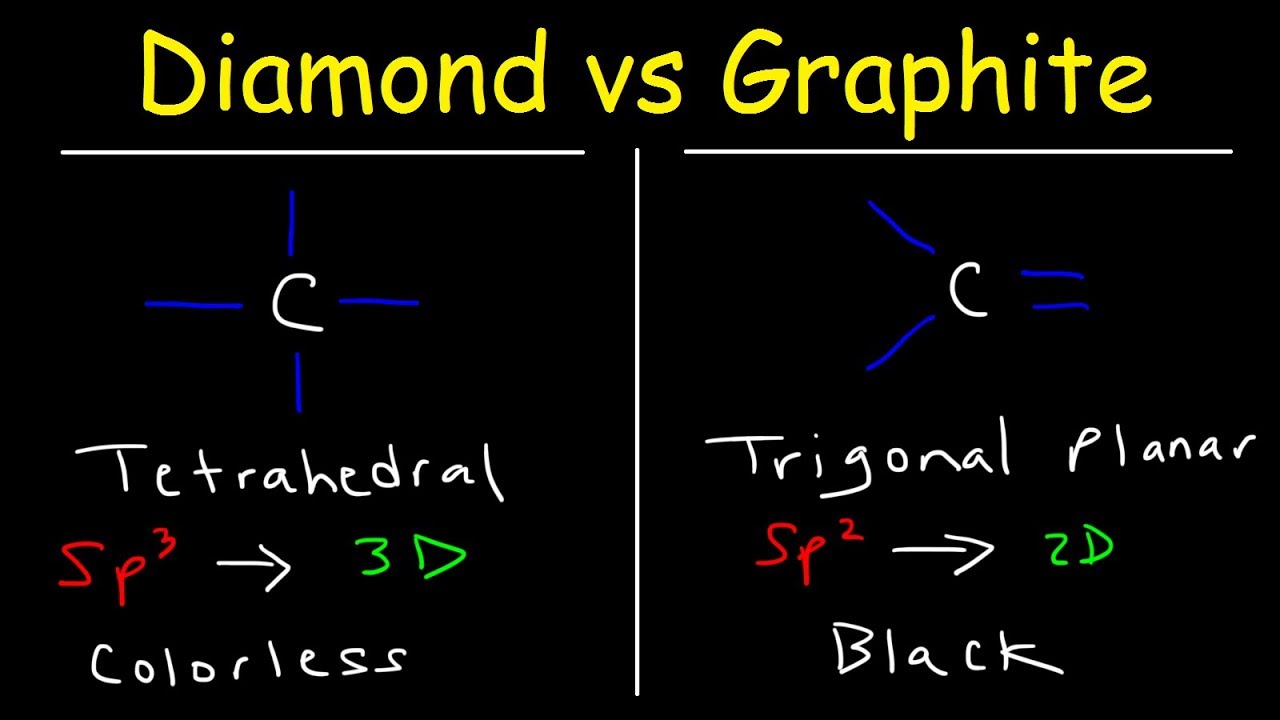
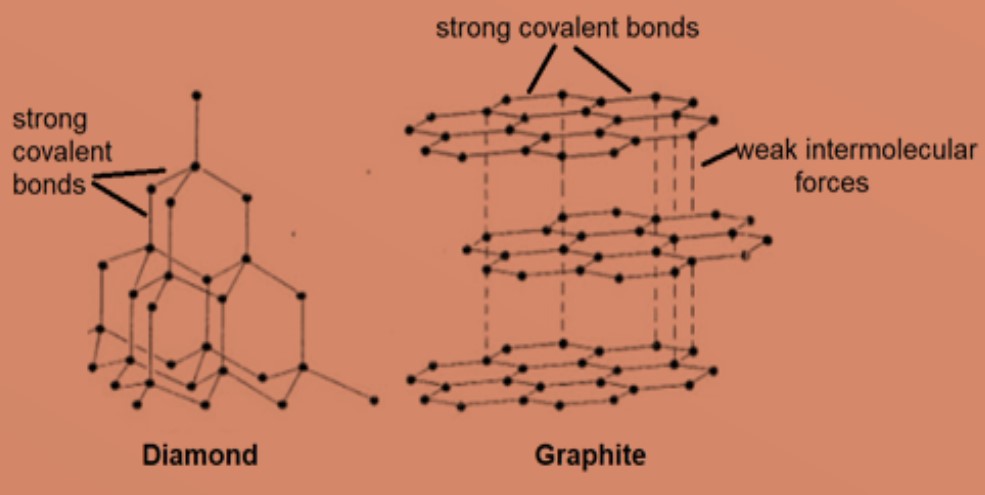
Compare the structures of Diamond and Graphite.

What is the formula for carbon graphite? - Quora
Structure of Diamond and Graphite, Properties - Basic Introduction
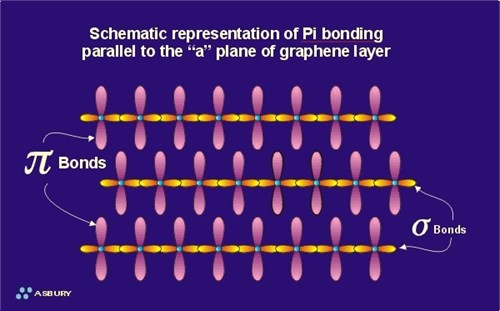
Graphite Structure
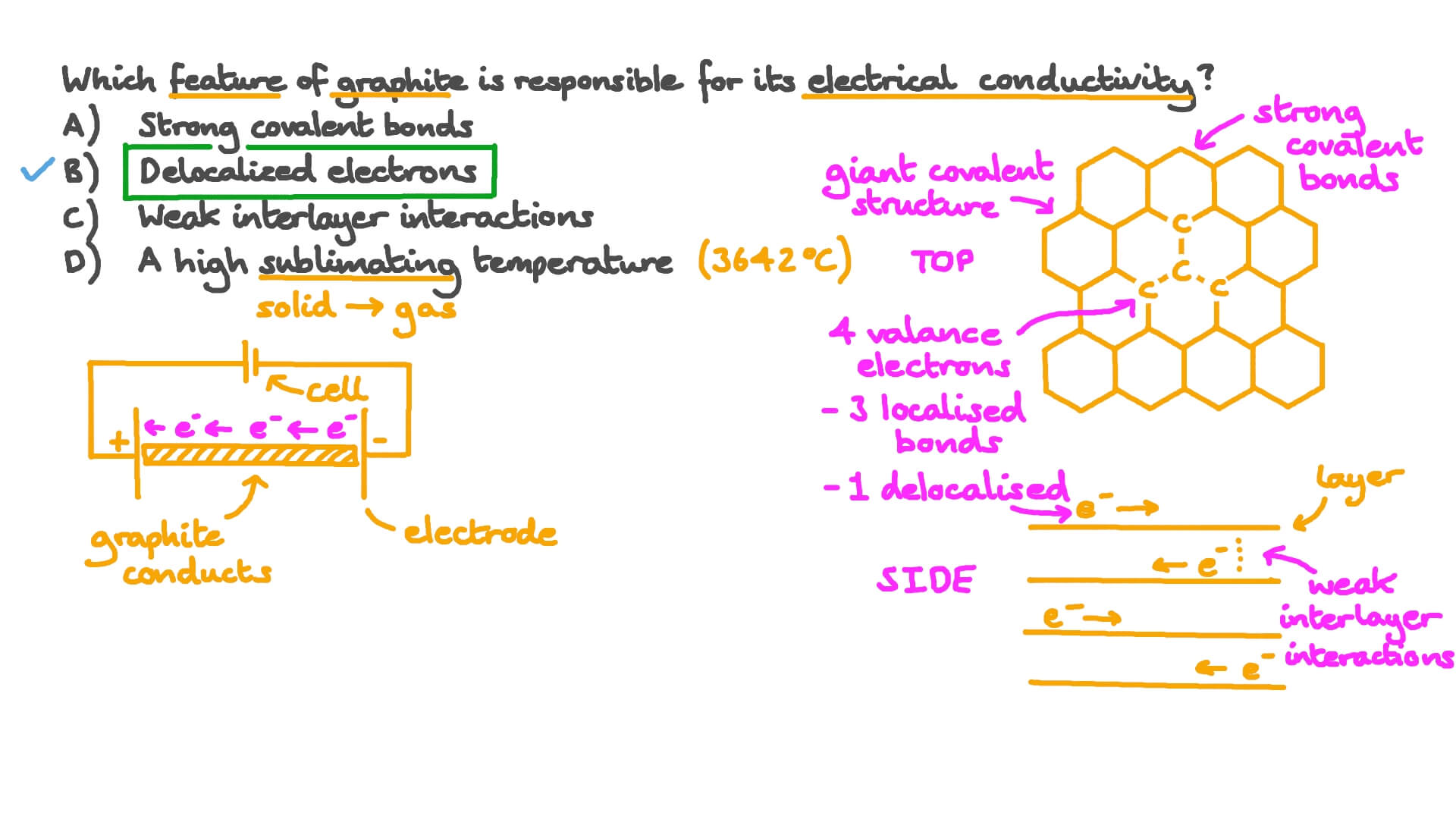
Question Video: Recalling Why Graphite Can Conduct Electricity

Difference between diamond and graphite is due to
.png)
a What is graphite? Of what substance is graphite made? b Describe the structure of graphite with the help of a labelled diagram. c Why is graphite a good conductor of electricity
Diamond and Graphite - Structure, Uses, Properties, Applications - GeeksforGeeks
14.4A: Graphite and Diamond - Structure and Properties - Chemistry LibreTexts

Hybridization of Graphite - Hybridization of Carbon in Graphite
macromolecules-or-giant-molecular-structures









